21
May
2023
- CR2O
- Press Releases
- C1, COVID-19, Dyadic, DYAI-100, SARS-CoV-2, Vaccines
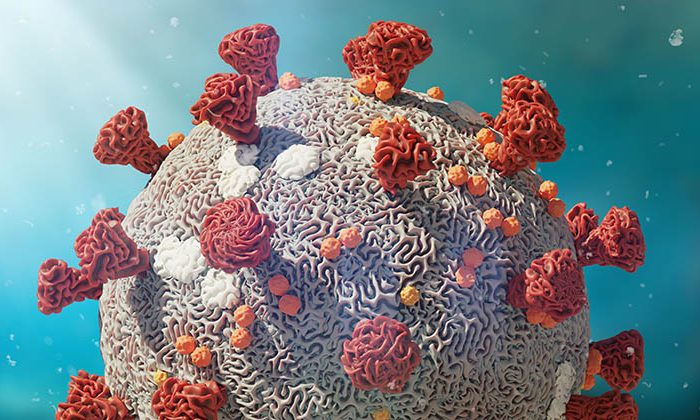
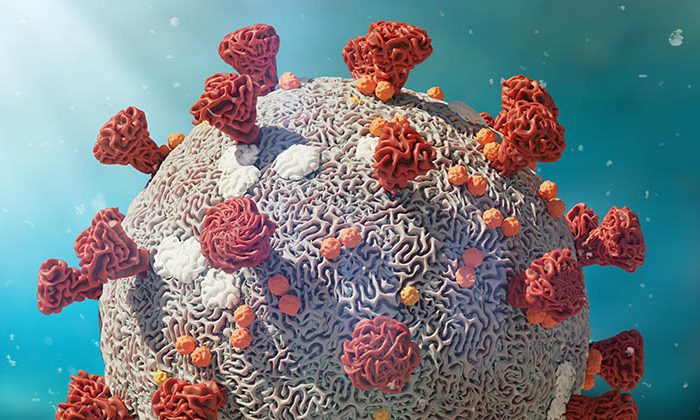
CR2O is proud to announce today it will manage and further support the clinical and preclinical development of DYAI-100, Dyadic’s C1 produced SARS-CoV-2-S-RBD vaccine candidate.
The first-in-human trial with DYAI-100 is expected to begin in H2 2021.
Dyadic International Inc., a global biotechnology company focused on further improving, applying and deploying its proprietary C1-cell protein production platform to accelerate development, lower production costs and improve the performance of biologic vaccines and drugs at flexible commercial scales, announced on March 18 2021 that the company’s initial C1 produced SARS-CoV-2-S-RBD vaccine candidate, DYAI-100, is moving towards an anticipated safety and preliminary efficacy first-in-human Phase I clinical trial. Dyadic has entered into a master services agreement with CR2O, as full-service global contract research organization specializing in vaccinology, to manage preclinical and clinical development of DYAI-100.
CR2O’s Chief Scientific Officer, Prof. Dr. Albert Osterhaus commented, “In response to the COVID-19 pandemic, pharmaceutical companies have developed vaccines within the unprecedented period of less than one year. To this end, and in close collaboration with strategic partners, they have implemented state-of-the-art technologies including the use of mRNA, viral vectors, and novel adjuvants. To effectively combat the COVID-19 pandemic worldwide, second generation variants of concern vaccines, produced at low cost and in large scale, are now urgently needed. The collaboration with Dyadic to use their highly-productive fungal C1-cell protein manufacturing system for this purpose appears to be a logical and promising way forward.”
CR2O’s CEO, Hadil Es-Sbai, added: “We are honoured to partner with Dyadic and its strategic partners in developing an affordable, scalable, protective and safe vaccine to combat this disease that continues to impact our everyday life”
For Dyadic’s original press release, please refer to Yahoo Finance.
Curious to learn more about CR2O’s full-service global CRO activities, or experience with vaccine trials?
Please, contact Ellemieke von Mauw:
Ellemieke von Mauw
Director Clinical Programs | Business Development
CR2O B.V.
T +31 (0)85 071 74 65 | M +31 (0)6 57 76 61 45 E | Ellemieke.vonmauw@cr2o.nl