24 AUG 2022
Larissa consortium reached milestone: Novel Rift Valley Fever vaccine dosed in Phase 1 human trial
The first healthy volunteer was dosed with hRVFV-4s, a novel live-attenuated Rift Valley fever vaccine. A clinical trial, run by the LARISSA[1] consortium and which started this week, will assess the safety, tolerability and immunogenicity of the vaccine candidate. “We are very proud and excited to have reached this milestone,” says Carine Punt, CEO of WUR Spin-out BunyaVax, the company managing the LARISSA consortium.
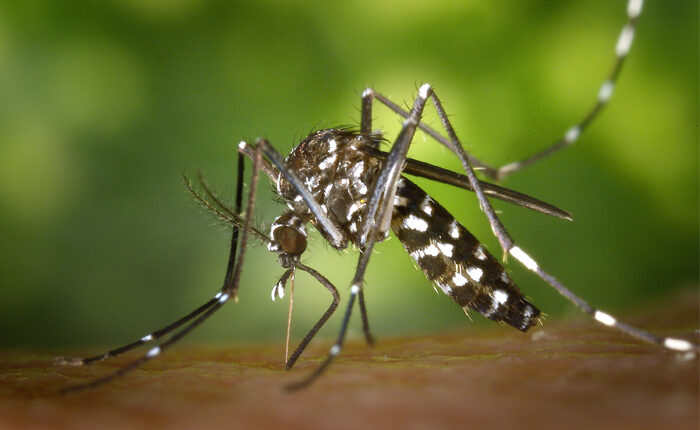
In this First-in-Human Clinical trial, designed as a dose escalation study, standard Phase I safety parameters as well as immunogenicity of the hRVFV-4s vaccine will be assessed in healthy adult volunteers. Following a single dose administration, the candidate vaccine is expected to generate a long-lasting immune response against Rift Valley fever, a severe and deadly disease predominantly transmitted by mosquitoes. The study will be performed at the Centre of Vaccinology in Belgium with CR2O as clinical operations partner.
Project
Currently, the only Rift Valley fever vaccines available are for use in livestock and none have been licensed for use in humans. The LARISSA consortium aims to address this deficiency. The consortium led by Wageningen Bioveterinary Research (WBVR), part of Wageningen University & Research, consists of six partners[2] and is financed by the Coalition for Epidemic Preparedness Innovations (CEPI), with support from the EU Horizon 2020 program.
One Health
“The vaccine virus, initially constructed by genetic engineering back in 2014, was found highly safe and efficacious in ruminants. Following years of optimizing production and developing a viable regulatory path for human use we can now make the first steps to protect humans from this emerging pathogen by a true One Health approach, in which a combination of human, animal and climatic variables are considered” states Paul Wichgers Schreur, Project Lead of the LARISSA consortium (WBVR).
“Our globally connected world has made us vulnerable to the rapid spread of zoonoses and the emergence of another pandemic disease is just a matter of time. Climate change, too, is also further compounding this risk, leading to an expansion of the range of disease-carrying animals, like mosquitoes, which can spread deadly diseases including Rift Valley fever”, said Dr Melanie Saville, Executive Director of Vaccine R&D at CEPI. “Given the potential for Rift Valley fever to cause significant disruption to health, economies and societies across sub-Saharan Africa and other regions, we can, and must, develop a safe and effective human vaccine against this viral threat to protect those people who are most at-risk and to improve global epidemic preparedness.”
About Rift Valley fever
Rift Valley fever virus was first identified in 1931 during an investigation into an outbreak among sheep on a farm in the Rift Valley of Kenya. Multiple outbreaks have since been reported across the African continent and on the Arabian Peninsula. Rift Valley fever virus mainly affects ruminants, leading to high mortalities among young animals and abortion storms in pregnant herds.
Humans become infected through close contact with infected animals or via mosquito bite. Despite the majority of infected humans present with mild flu-like symptoms the infection may develop into a life-threatening disease in about 1-2% of infections. As susceptible mosquitoes are expanding their territory, there is a concern that the virus will spread to naive regions in the near future. In view of the epidemic threat posed by this disease, the WHO has classified it as a priority pathogen in need of urgent R&D investment.
[1] LARISSA is the acronym for Live-attenuated Rift Valley fever vaccine for single-shot application
[2] Partners of LARISSA are: WBVR/WUR, IDT Biologika GmbH, Research Centre for Emerging Infections and Zoonoses (RIZ)/TiHo, CR2O BV, CEVAC-CTU UZGent/CEVAC-LAB UGent, BunyaVax BV.
